Vous ressentez parfois une faim soudaine et impérieuse, ce besoin pressant de manger qualifié de « foncedalle » ? Ce phénomène, souvent trivialisé, cache en réalité un mécanisme complexe. Hormones, réactions physiologiques

Spécialiste de l’arthrose du genou : comment il peut vous aider ?Spécialiste de l’arthrose du genou : comment il peut vous aider ?
Si vous souffrez de l’arthrose du genou, se rapprocher d’un spécialiste est important pour comprendre et agir efficacement. Ce professionnel, par son expertise, établit un diagnostic méticuleux et guide chaque

Conseils de santé en ligne : Avantages et limitesConseils de santé en ligne : Avantages et limites
Dans un monde où la technologie numérique occupe une place prépondérante, l’accès à des conseils de santé en ligne est devenu omniprésent. De nombreux individus recherchent désormais des informations sur

Collagène marin : votre allié anti-âge et pour une santé globale amélioréeCollagène marin : votre allié anti-âge et pour une santé globale améliorée
Le collagène marin s’affirme comme une source incontournable dans la lutte contre le vieillissement cutané et l’amélioration de la santé. Indispensable pour maintenir l’élasticité et la jeunesse de la peau,

Trouvez les meilleurs ophtalmologues autour de vous en un clic!Trouvez les meilleurs ophtalmologues autour de vous en un clic!
Dans le choix délicat d’un ophtalmologue, la proximité n’est que la première des préoccupations. Comment être sûr de l’expertise et du professionnalisme du spécialiste qui prendra en charge la santé

Les critères de qualité et les garanties à vérifier pour trouver des coussins orthopédiques dosLes critères de qualité et les garanties à vérifier pour trouver des coussins orthopédiques dos
Trouver le coussin orthopédique dorsal parfait peut être un défi, surtout lorsque l’on considère l’importance cruciale de ce support pour la santé et le bien-être. Les critères de qualité et

Tout savoir sur la cryothérapie le mans : bienfaits et fonctionnementTout savoir sur la cryothérapie le mans : bienfaits et fonctionnement
Soulagement des douleurs, amélioration du sommeil et effets rajeunissants : la cryothérapie suscite un intérêt croissant au Mans. Cette thérapie par le froid, non seulement apaisante mais aussi revitalisante, s’offre

Pervers narcissique : les points pour le reconnaître facilementPervers narcissique : les points pour le reconnaître facilement
Dans une société où les relations humaines sont de plus en plus complexes, le terme de pervers narcissique est souvent évoqué. Derrière ces mots se cache une réalité bien sombre,

Séances d’ostéopathie : astuces pour optimiser votre remboursement et réduire vos dépensesSéances d’ostéopathie : astuces pour optimiser votre remboursement et réduire vos dépenses
Mal de dos, tensions musculaires, troubles digestifs… l’ostéopathie promet soulagement et bien-être. Encore faut-il maîtriser les rouages du remboursement pour ne pas alourdir votre budget. Entre choix de mutuelle et
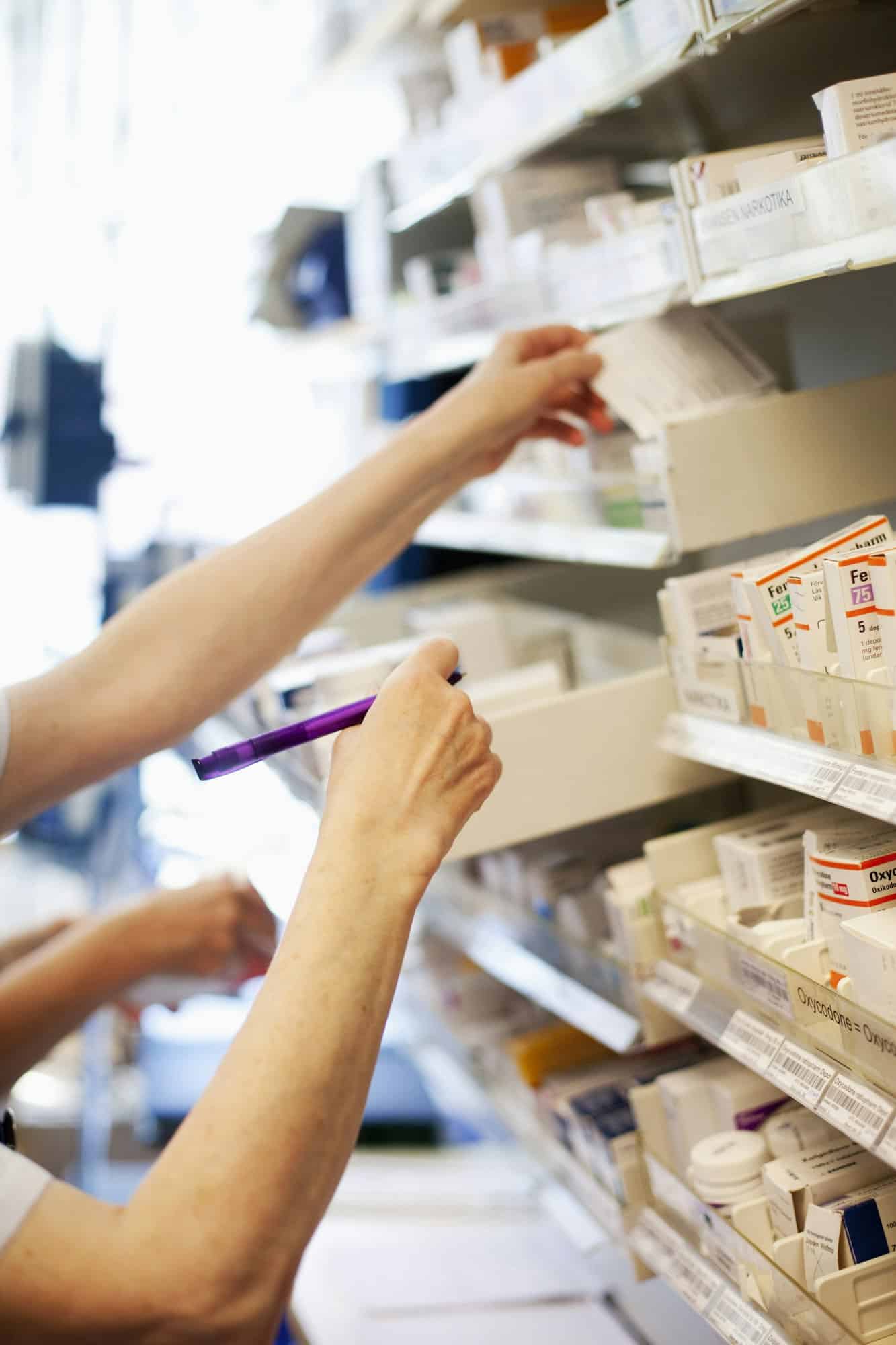
Les points de repère de la santé : Les grandes pharmacies de ParisLes points de repère de la santé : Les grandes pharmacies de Paris
À Paris, les pharmacies ne se contentent pas de dispenser des soins; elles incarnent également un patrimoine singulier fusionnant histoire, architecture et tradition. Des enseignes séculaires aux façades imposantes, ces
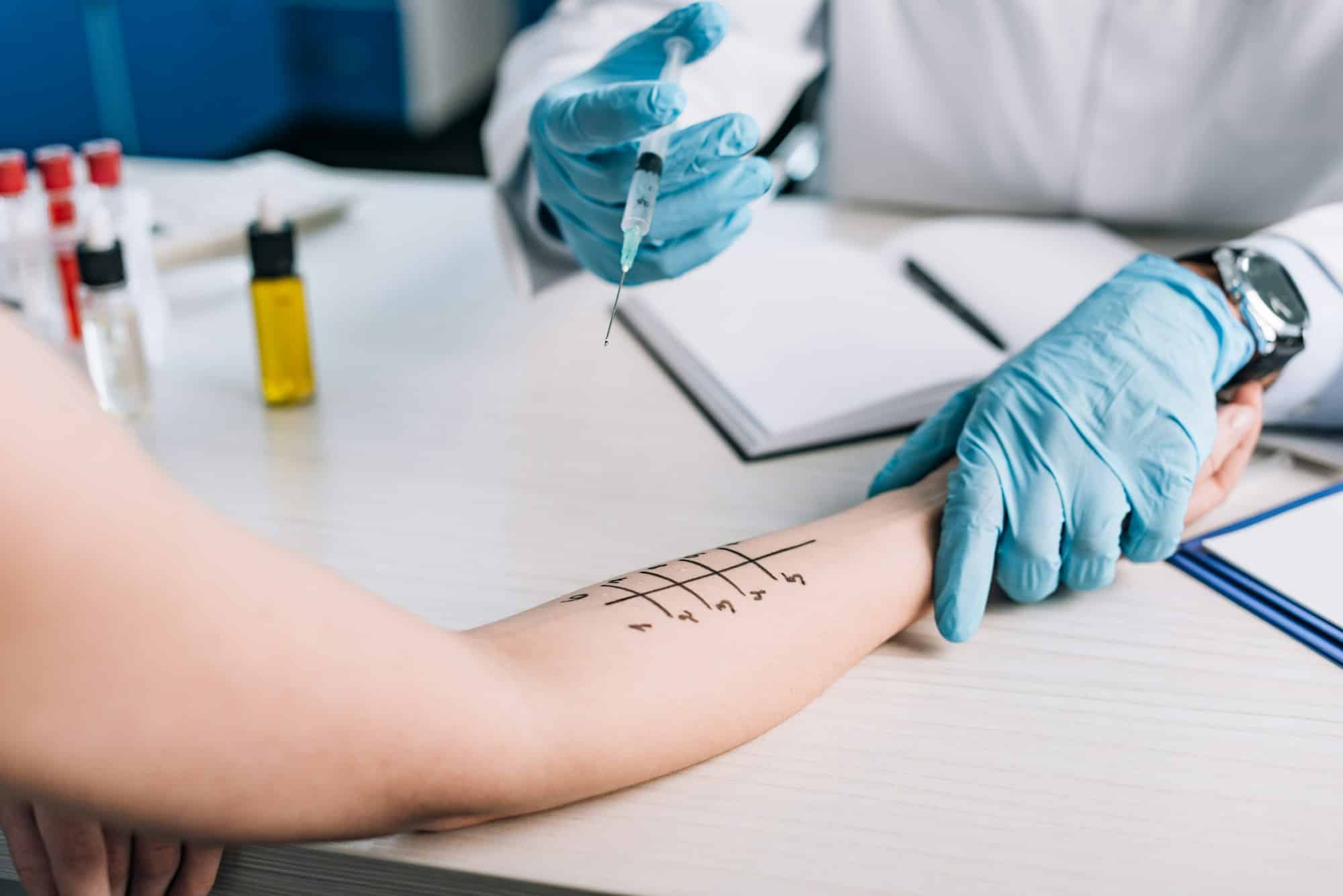
Pourquoi se rendre auprès d’un allergologue à Nantes ?Pourquoi se rendre auprès d’un allergologue à Nantes ?
À Nantes, la lutte contre les allergies se personnifie. Reconnaître à temps les symptômes qui alertent et solliciter un diagnostic précis demeurent les premiers pas vers un soulagement durable. Consulter

Sécurité infantile : Protéger votre bébé des chutes et des blessures à la têteSécurité infantile : Protéger votre bébé des chutes et des blessures à la tête
La sécurité des bébés est une préoccupation majeure pour tous les parents et soignants. Les chutes font partie des accidents domestiques les plus courants chez les jeunes enfants, pouvant entraîner

L’essor des maisons de santé dans les Landes : Un nouveau modèle de santé accessibleL’essor des maisons de santé dans les Landes : Un nouveau modèle de santé accessible
Face à un défi sanitaire persistant en zone rurale, les maisons de santé des Landes se multiplient, promettant un accès facilité à des soins primaires de qualité. Ces structures innovantes

My Energy Pump : un booster pour votre corpsMy Energy Pump : un booster pour votre corps
Améliorer sa performance physique présente de nombreux avantages pour la santé et le bien-être. En renforçant son corps et en augmentant son endurance, on gagne en condition physique générale, ce

Quels sont les services et les compétences d’un dentiste à Niederhausbergen ?Quels sont les services et les compétences d’un dentiste à Niederhausbergen ?
Dans la quête du bien-être et de la confiance en soi, le sourire joue un rôle central. À Niederhausbergen, trouver le dentiste idéal pour entretenir ce précieux atout va au-delà

L’impact de l’AAH : Infos handicap essentielle sur le soutien financier et socialL’impact de l’AAH : Infos handicap essentielle sur le soutien financier et social
L’allocation aux adultes handicapés (AAH) est un élément important de l’accompagnement des personnes handicapées en France. Ces prestations vont bien au-delà d’un simple soutien financier et constituent un facteur important

Adoptez une routine de soins bio pour votre bébé : pourquoi et comment ?Adoptez une routine de soins bio pour votre bébé : pourquoi et comment ?
Lorsque vous êtes enceinte, prendre soin de vous devient une priorité. Vous voulez ce qu’il y a de mieux pour vous et votre bébé, mais il peut être difficile de

Les services d’urgence médicale à Marseille : une vue d’ensembleLes services d’urgence médicale à Marseille : une vue d’ensemble
Marseille, ville dynamique de la côte méditerranéenne, se distingue par ses ruelles pittoresques et son patrimoine culturel riche. Cependant, derrière cette beauté, la cité phocéenne doit relever le défi complexe

Critères pour choisir le meilleur site de produits à base de chanvreCritères pour choisir le meilleur site de produits à base de chanvre
Les produits de CBD tendent à se démocratiser de nos jours. Grâce à leur vente en ligne, ils sont accessibles à un public beaucoup plus large. Par ailleurs, les achats

Que savez-vous des différents types de professions de la santé ?Que savez-vous des différents types de professions de la santé ?
Le domaine de la santé est assez vaste et l’on y distingue plusieurs corps. Chaque personnel du corps de la santé a quand même un rôle bien défini. Nombreux sont
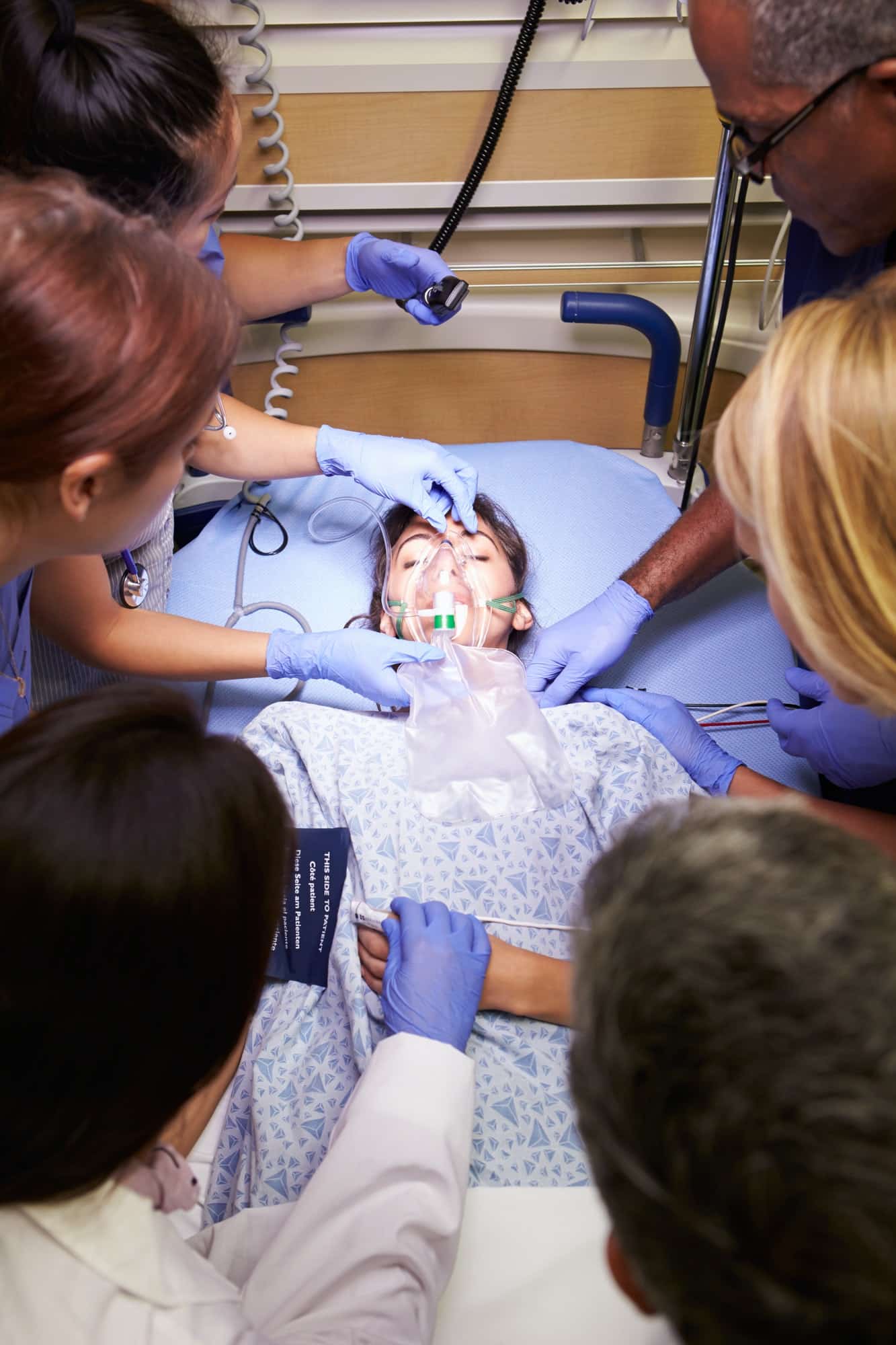
Que faire en cas d’urgence médicale ?Que faire en cas d’urgence médicale ?
Les situations d’urgence médicale, on ne s’y attend jamais. Lorsque celles-ci surviennent, il n’est pas vraiment évident de savoir quoi faire. Toujours est-il qu’il est préférable de savoir à l’avance

Quels avantages d’apprendre le magnétisme dans une école spécialisée ?Quels avantages d’apprendre le magnétisme dans une école spécialisée ?
Le magnétisme sert à soulager les personnes qui souffrent des maux, notamment d’ordre spirituel. Pour certains spécialistes, c’est un véritable don qu’ils mettent au service de leurs proches. Quels avantages

Les considérations principales sur l’utilisation du CBD pendant la grossesseLes considérations principales sur l’utilisation du CBD pendant la grossesse
Les études sur l’utilisation du CBD pendant la grossesse soulèvent des questions critiques quant à ses effets potentiels sur la santé maternelle et le développement du fœtus. Cette question alimente

Visual CBD : comment simplifie-t-il votre exploration des options CBD ?Visual CBD : comment simplifie-t-il votre exploration des options CBD ?
En raison de la croissance rapide du marché du CBD, il est parfois difficile pour de nombreux consommateurs de trouver le produit idéal. À cet égard, Visual CBD fait figure

Compléments alimentaires contre l’arthrose : lequel choisir ?Compléments alimentaires contre l’arthrose : lequel choisir ?
À partir d’un certain âge, la plupart des gens se plaignent de douleurs articulaires. Cependant, il peut s’agir d’une grave affection articulaire appelée arthrose. L’arthrose peut affecter considérablement votre vie

Quels sont les bienfaits de l’hypnose régressive ?Quels sont les bienfaits de l’hypnose régressive ?
L’hypnose régressive a émergé comme une pratique intrigante. Elle offre un regard unique sur les méandres de l’esprit humain et de ses expériences passées. Alors que certains peuvent être sceptiques

Peut-on boire du café au lait pendant le jeûne intermittent ?Peut-on boire du café au lait pendant le jeûne intermittent ?
Le jeûne intermittent est une pratique qui connaît un essor car elle présenterait certains bienfaits pour la santé comme la réduction du risque de diabète ou de maladies cardiovasculaires. Il
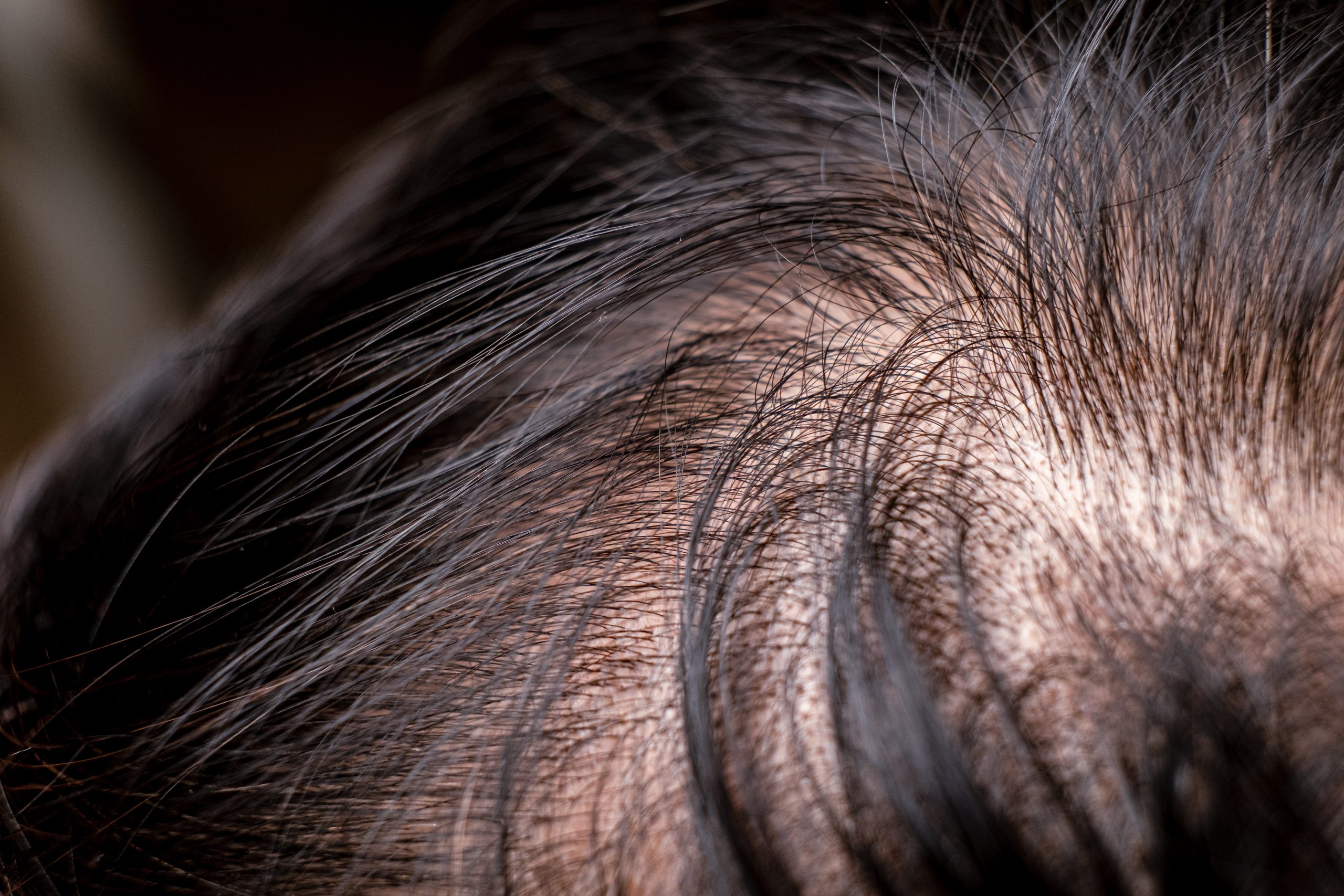
Chimiothérapie et perte de cheveux : comment choisir sa prothèse capillaire ?Chimiothérapie et perte de cheveux : comment choisir sa prothèse capillaire ?
L’alopécie, qui se caractérise par la chute des cheveux, peut être une épreuve difficile pour les femmes qui subissent une chimiothérapie. Heureusement, il existe des solutions, telles que l’utilisation de

Comment garantir une expérience optimale pour les personnes âgées lors de l’achat d’un téléphone portable ?Comment garantir une expérience optimale pour les personnes âgées lors de l’achat d’un téléphone portable ?
L’achat d’un téléphone portable pour une personne âgée est une démarche qui va au-delà du simple choix d’un modèle. Il s’agit de prendre en compte les besoins spécifiques de l’utilisateur.

Quels sont les bienfaits de l’harpagophytum bio pour votre santé ?Quels sont les bienfaits de l’harpagophytum bio pour votre santé ?
L’harpagophytum bio, aussi bien connu sous le nom de « griffe du diable » en raison de son apparence distinctive, est une plante originaire d’Afrique, principalement du sud du continent. Depuis des
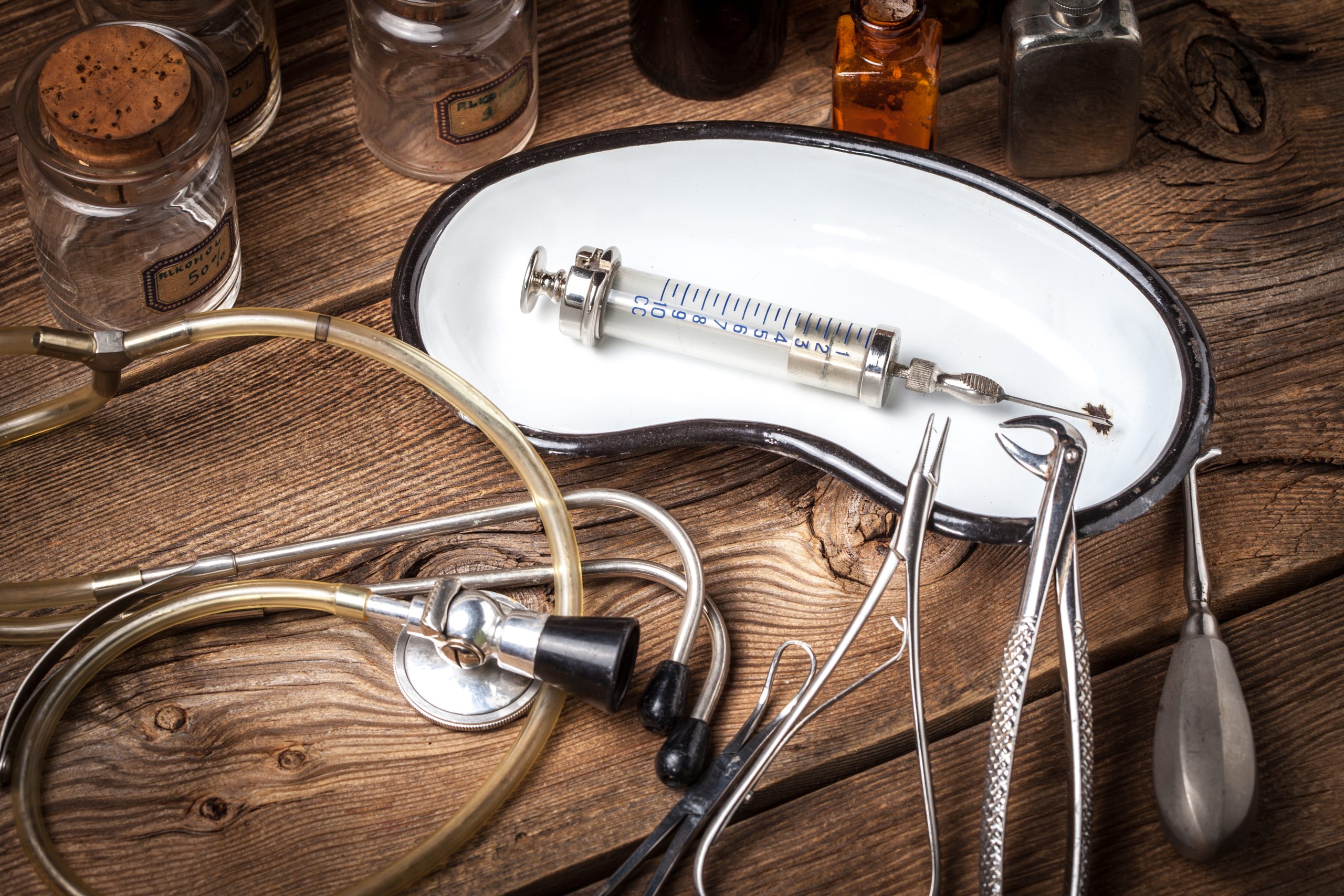
Les types d’instrument médical et ses caractéristiquesLes types d’instrument médical et ses caractéristiques
Chaque catégorie d’instrument médical est dédiée à créer des solutions avancées pour les professionnels de la santé ainsi que pour les établissements médicaux. Il existe une gamme complète d’instruments médicaux

Santé en urgence : la pharmacie de garde répond à vos appels jour et nuitSanté en urgence : la pharmacie de garde répond à vos appels jour et nuit
À tout moment, que ce soit la nuit ou les jours fériés, des situations médicales d’urgence peuvent survenir. L’expertise des pharmacies de garde, ouvertes tous les jours toute la journée,

Conseils pour trouver rapidement une pharmacie de garde en urgenceConseils pour trouver rapidement une pharmacie de garde en urgence
La première raison de solliciter les services d’une pharmacie de garde en urgence est le besoin pressant de médicaments en dehors des heures d’ouverture normales. Les situations où une ordonnance

Quels sont les différents types de bois utilisés pour fabriquer des saunas infrarouges ?Quels sont les différents types de bois utilisés pour fabriquer des saunas infrarouges ?
Les saunas infrarouges sont de plus en plus populaires notamment pour leurs bienfaits sur la santé. Ils permettent de se détendre, de se relaxer et d’éliminer les toxines du corps.

les bienfaits de la marche pour les seniorsles bienfaits de la marche pour les seniors
La marche fait partie de ces activités sportives qui ont le mérite d’être accessibles à tous, quel que soit l’âge ou le niveau de condition physique. Elle présente de nombreux

Les nouvelles directives pour prévenir les maladiesLes nouvelles directives pour prévenir les maladies
Chers lecteurs, en cette période exceptionnelle marquée par le virus du COVID-19, la question de notre santé est plus que jamais au cœur des préoccupations. Dans un contexte où les

Les bienfaits de l’eau citronnée pour la perte de poidsLes bienfaits de l’eau citronnée pour la perte de poids
Il est 10h du matin, vous êtes assis à votre table de cuisine, un verre d’eau citronnée à la main. Vous le regardez, interrogez le liquide transparent et citronné avec

conseils pour préserver sa santé mentale après 60 ansconseils pour préserver sa santé mentale après 60 ans
Nous vieillissons tous et cela peut être une période de grands changements. En vieillissant, nous pouvons être confrontés à des défis physiques et mentaux. Les problèmes de santé mentale chez

les nouvelles études sur les effets de l’alimentation sur la santéles nouvelles études sur les effets de l’alimentation sur la santé
Effectuez une navigation sur (ou une recherche dans) n’importe quelle page web d’information médicale, et vous tomberez inévitablement sur un article ou une étude qui souligne l’importance de l’alimentation pour

quels sont les symptômes de l’asthme et comment le gérer au quotidien ?quels sont les symptômes de l’asthme et comment le gérer au quotidien ?
Chaque année, l’asthme touche des millions de personnes à travers le monde. Cette maladie respiratoire chronique peut survenir à tout âge et affecte la qualité de vie des personnes atteintes.

Comment développer une routine de gratitude pour cultiver le bien-être au quotidienComment développer une routine de gratitude pour cultiver le bien-être au quotidien
Dans le tourbillon de nos vies trépidantes, la quête du bonheur et de la sérénité peut parfois sembler hors de portée. Entre les obligations professionnelles, les défis personnels et l’avalanche

les meilleures astuces pour une grossesse en pleine formeles meilleures astuces pour une grossesse en pleine forme
La grossesse est une période particulière de la vie d’une femme. Elle est à la fois fascinante et épuisante, et nécessite un niveau de soins et d’attention particulier pour assurer

Les conseils pour éviter la reprise de poids après un régimeLes conseils pour éviter la reprise de poids après un régime
Se lancer dans un régime alimentaire pour perdre du poids est une décision importante, mais souvent le plus grand défi réside dans la période qui suit la fin du régime.

comment soulager les douleurs liées à la fibromyalgie ?comment soulager les douleurs liées à la fibromyalgie ?
Vous souffrez de douleurs persistantes, et on vous a diagnostiqué la fibromyalgie ? Cette maladie, caractérisée par des douleurs musculaires diffuses et un trouble du sommeil, peut véritablement bouleverser votre

comment prévenir et traiter les problèmes de circulation pendant la grossessecomment prévenir et traiter les problèmes de circulation pendant la grossesse
La grossesse est une période de grande joie et d’anticipation pour la plupart des femmes. Cependant, elle peut aussi être accompagnée de certains désagréments et défis, notamment ceux liés à

Les bienfaits de la nature sur le bien-être et la santé mentaleLes bienfaits de la nature sur le bien-être et la santé mentale
Laissez-moi vous raconter un secret. Non, ce n’est pas le dernier gadget de la technologie ni une nouvelle tendance de mode. C’est quelque chose de beaucoup plus profond et plus

Tout ce qu’il faut savoir sur la formation d’un coach en nutritionTout ce qu’il faut savoir sur la formation d’un coach en nutrition
Le cours d’éducateur en nutrition est un programme de formation qui permet aux personnes de devenir des experts en matière de nutrition, de santé et de bien-être. Il permet d’acquérir

Statistiques et des informations clés sur l’utilisation et la consommation du cannabidiolStatistiques et des informations clés sur l’utilisation et la consommation du cannabidiol
Nombreuses sont les études effectuées ayant prouvé l’efficacité de la prise du CBD pour lutter contre le stress. Ce produit est réputé pour ses effets relaxants et ses propriétés thérapeutiques.

Bain pour les pieds : une pratique essentielle pour votre bien-êtreBain pour les pieds : une pratique essentielle pour votre bien-être
Les pieds constituent un point stratégique pour le corps humain. En effet, son rôle est similaire à celui de la fondation d’une maison. Plus elle est bien entretenue, meilleure est

Cancer du sein et alimentation : ce que vous devez savoirCancer du sein et alimentation : ce que vous devez savoir
Le cancer du sein est loin d’être une pathologie anodine. Il faut toujours le prendre au sérieux parce que ses conséquences sont terriblement néfastes tant sur la santé, ou sur

